
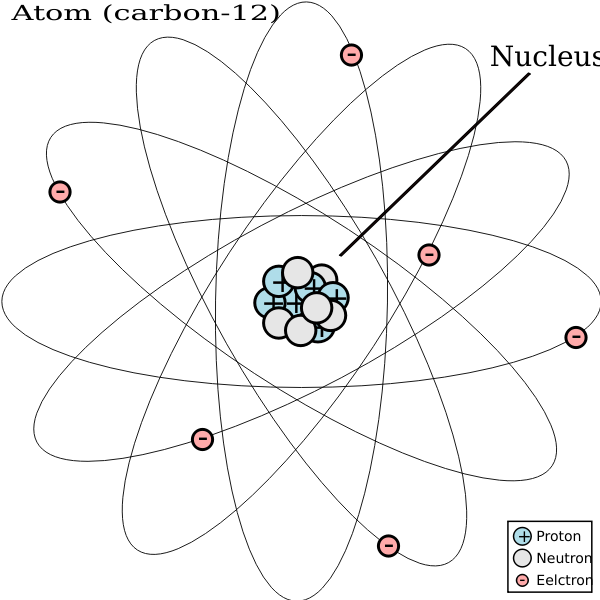
Where Carbon 12 has six protons and six neutrons, Carbon 14 has six protons and eight neutrons. Another main difference is the number of protons and electrons. This instability occurs due to the different number of atoms present in the latter. 2020 The key is in the rock’s atomic composition organisms that use sunlight for energy incorporate a lighter version of carbon, called carbon 12, over the heavier carbon 13. Carbon 12 is the most stable isotope of Carbon, while carbon 14 is the most unstable one. 2020 By controlling the magnetic field and other conditions inside the cannon, the researchers cause the carbon 14 atoms to veer away from the carbon 12 atoms and other elements. Scott Hershberger, Scientific American, 4 Aug. 2020 Chemically, carbon 14 behaves exactly like its stable siblings ( carbon 12 and carbon 13), allowing plants to absorb it during photosynthesis and then pass it up the food chain. So, our amount is 12 grams multiplied by one mole. You might see this written as is equal to divided by capital. We can work out the number of moles of atoms in 12 grams of carbon-12 by taking the mass and dividing by the molar mass. Scott Hershberger, Scientific American, 4 Aug. Therefore, the mass per mole of carbon-12 is 12 grams per mole, meaning moles of carbon-12 atoms. 2021 So researchers compare the amount of carbon 14 with the levels of carbon 12 and carbon 13 to determine how much time has passed since an organism perished. Carbon 12 was established in 2008, initiating a comprehensive, firmly global, program of institution-grade artists. Jonathan O'Callaghan, Scientific American, 10 Nov. Carbon-12: with 6 protons and 6 neutrons and an atomic mass of 12 Carbon-14: with 6 protons and 8 neutrons, and an atomic mass of 14.
2022 But if Perseverance’s sample is found to contain not only methane but methane rich in an isotope called carbon 12, that could change. Clara Moskowitz, Scientific American, 15 June 2023 Carbon has two stable isotopes, measured as either carbon 12 or carbon 13. is produced by thermal neutrons from cosmic radiation in the upper atmosphere, and is transported down. are stable, occurring in a natural proportion of approximately 93:1. There are three naturally occurring isotopes of carbon: 12, 13, and 14. Carbon-12 provides a road map for growth, increases brand value, drives revenue and stays ahead of the curve, setting the standards for innovative companies. Recent Examples on the Web Measuring the extent to which multiple carbon 13 atoms (a rare, stable form of carbon with an extra neutron) replace carbon 12 (the most common form of carbon) in a particular molecule, for instance, can tell researchers about the temperature when the compound formed. Natural isotopes edit Main articles: Carbon-12, Carbon-13, and Carbon-14.


 0 kommentar(er)
0 kommentar(er)
